In February 2022, LG Chem announced their plans to become a global science company, and revealed their intention to increase revenue ten times by 2030, from the current $2.2 billion (KRW 3 trillion) to $22.6 billion (KRW 30 trillion), with three new growth engines; eco-friendly materials, battery materials, and globally-innovative new drugs.
In their bid to become a global pharmaceutical company, LG Chem has secured 19 pipelines for global drugs in clinical trial stages phase 1 or above, and has accelerated R&D efforts. Through the application of novel AI models and by endeavoring to develop at least four new FDA-approved drugs by 2030, LG Chem intends to secure a position within the top 30 global oncology-focused pharmaceutical companies. After unveiling their plans to develop new drugs for the global market, LG Chem is increasing their presence by obtaining the FDA’s approval for clinical test plans, and through the acquisition of a bio company. Learn more about LG Chem’s moves in the global bio market.
From new obesity drug that received additional ODD by the US FDA to new gout drug that obtained approval for global phase 3 clinical trials

In June 2022, LG Chem’s new drug for genetic obesity treatment received additional Orphan Drug Designation (ODD) from the US FDA. The new drug, LB54640, is orally administered once a day targeting the pathway of a gene, MC4R (Melanocortin 4 Receptor), which controls appetite. When a working pathway of MC4R malfunctions, a feeling of hunger continues and thus worsens obesity. LB54640 works directly on MC4R protein that sends a signal of satiety, and induces suppression of appetite. LG Chem recently completed clinical phase 1 study of LB54640 in the US for healthy overweight adults without genetic defects, and plans to conduct global phase 2 and 3 clinical trials for genetic obesity patients with LEPR or POMC deficiency starting this year.
LG Chem is also developing Tigulixostat, a new drug for gout treatment, and plans a global phase 3 clinical trial, hoping to bring the new drug to gout patients around the world as quickly as possible. In September 2022, LG Chem submitted a plan for the second round of phase 3 clinical trial of Tigulixostat to the US FDA, and obtained approval in November 2022. The new clinical trial is conducted with a control group of patients who take allopurinol, which is a primary selective ingredient for gout treatment. The clinical trial will be conducted for 2,600 adult gout patients with hyperuricemia for 12 months to observe long-term administration stability, the effect of lowering blood levels of uric acid, and reducing gout attacks and tophus. Based on distinguished outcomes of the clinical trials, LG Chem endeavors to sharpen their competitive edge in the global bio market.
Acquisition of an oncology-focused US biopharmaceutical company
that has FDA-approved new drugs

LG Chem successfully acquired AVEO Pharmaceuticals(“AVEO”), an innovative US anti-cancer pharmaceutical company that has an FDA-approved new drug for renal cancer treatment. Established in 2002 in Boston, Massachusetts, the company focuses on oncology markets in terms of clinical development, drug approval, sales, and marketing. In 2021, AVEO obtained FDA approval for their renal cancer drug, Fotivda, and the company’s revenues are expected to grow. By acquiring AVEO in a $571 million deal, LG Chem took over 100% of AVEO shares.
In the US, systems regarding medical insurance, drug fees, and drug circulation are widely different from Korea, and localized commercialization capacity is required from the early stage of drug development. It is a difficult market to pioneer, but business for anti-cancer drugs can be managed with a sales unit focusing on a few oncology-dedicated medical institutions. In addition to Fotivda, AVEO has three more pipelines for cancer drugs in the stage of clinical development including Ficlatuzumab, a drug for head and neck cancer treatment for which a phase 3 clinical trial is underway. If all three drugs are successfully developed, they are expected to obtain FDA approvals in the next 30 years. With the successful acquisition deal, LG Chem instantly secured a presence in the US market for anti-cancer drug commercialization. The company also gained a foothold to develop and commercialize new drugs in the US, the world’s largest market for pharmaceutical products.
With continued R&D efforts, LG Chem stands at the center of the global bio industry
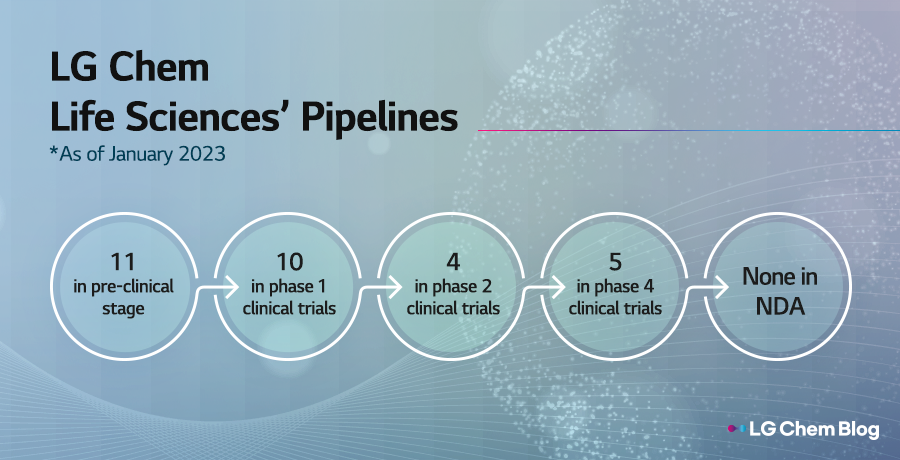
Until now, LG Chem Life Sciences division has focused on vaccines, diabetes treatment, cosmetic fillers, and growth hormones. In February 2022, the company announced a plan to foster three new growth engines, and shortly after that, their new drug for obesity treatment received an ODD from the US FDA. LG Chem’s plan to conduct a global phase 3 clinical trial for a new drug for gout treatment was approved, and the company also acquired an oncology-focused US biopharmaceutical company, making headways in increasing presence in the global bio market. LG Chem absorbed AVEO’s capacity for drug commercialization and clinical development, and expects to earn revenues worth $1.5 billion (appr. KRW 2 trillion) from the Life Sciences business.




There are no comments yet! Be the first to let us know your thoughts!